so42- lewis structure|Iba pa : Tuguegarao Learn how to draw the Lewis structure of sulfate ion (SO42-) with formal charge calculation and VSEPR theory. Find out the molecular geometry, hybridization, . Hanime1.me 帶給你最完美的H動漫、H動畫、裏番、里番、成人色情卡通片的線上看體驗,絕對沒有天殺的片頭廣告! H anime1 . me 裏番 新番預告 泡麵番 Motion Anime 3D動畫 同人作品 MMD Cosplay H漫畫 無碼黃油 account_circle search cast
so42- lewis structure,A step-by-step explanation of how to draw the SO4 2- Lewis Dot Structure (Sulfate ion). For the SO4 2- structure use the periodic table to find the total number of valence electrons .more. Learn how to draw the Lewis structure of sulfate ion (SO42-) with formal charge calculation and VSEPR theory. Find out the molecular geometry, hybridization, .
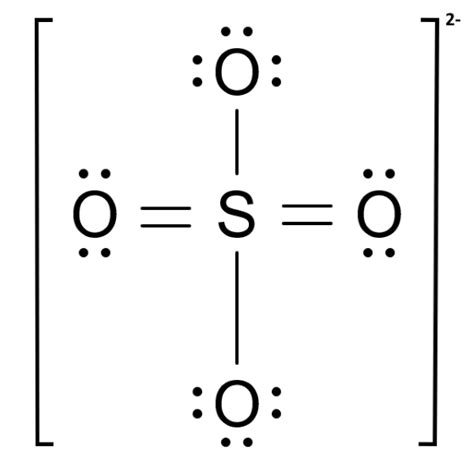
A step-by-step explanation of how to draw the Sulfate Ion Lewis Dot Structure (SO42- ). We'll also look at the molecular geometry, bond angles, electron geometry, and resonance for the. This chemistry video explains how to draw the lewis structure of the sulfate ion SO4 2-.How To Draw Lewis Structures: https://www.youtube.com/wat.Lewis Structure for SO4 2- (Sulfate Ion) Commonly Tested Lewis Structures. We draw Lewis Structures to predict: -the shape of a molecule. -the reactivity of a molecule and .The Lewis structure for SO 42- is requires you to place more than 8 valence electrons on Sulfur (S). You might think you've got the correct Lewis structure for SO 4 at first. . SO42- Lewis Structure, Hybridization, Bond Angle and Molecular Geometry. SO42- is a chemical name for the sulfate ion. It comprises one Sulphur atom, four Oxygen atoms, and a charge of -2. .

Learn how to draw the Lewis structure of SO4 2- (sulfate ion), a chemical species with intriguing properties. Follow the steps to determine valence electrons, choose the .
so42- lewis structure Iba paLearn how to draw the Lewis structure of SO4 2- (sulfate ion), a chemical species with intriguing properties. Follow the steps to determine valence electrons, choose the . The SO42- Lewis structure depicts the molecular arrangement of sulfate, which consists of one sulfur atom and four oxygen atoms. The structure has two double bonds and two single bonds . Sulfur make six bonds in this Lewis Structure. Two of the oxygens are single-bonded and two are double-bonded. The reason is FORMAL CHARGE and the fact tha.
so42- lewis structure Sulfur make six bonds in this Lewis Structure. Two of the oxygens are single-bonded and two are double-bonded. The reason is FORMAL CHARGE and the fact tha.
Hi Everyone! For today’s video, we are going to do SO42- Lewis Structure. It is a chemical formula for Sulfate ions. To determine its Lewis Structure, we fir. 5 Steps to Draw the Lewis Structure of SO4 2- ion Step #1: Calculate the total number of valence electrons. Here, the given ion is SO4 2-.In order to draw the lewis structure of SO4 2-ion, first of all you have to find the total number of valence electrons present in the SO4 2-ion. (Valence electrons are the number of electrons present in the . Steps of drawing SO4 2- lewis structure Step 1: Find the total valence electrons in SO4 2- ion. In order to find the total valence electrons in SO4 2- ion, first of all you should know the valence electrons present in sulfur atom as well as oxygen atom. (Valence electrons are the electrons that are present in the outermost orbit of any atom.)
Lewis Dot of the Sulfate Ion. SO 42-. Back. 70 More Lewis Dot Structures. S does not follow the octet rule. It will hold more than 8 electrons. Sulfur having valence electrons in the 3rd energy level, will also have access to the 3d sublevel, thus allowing for more than 8 electrons. Elements in the first 2 periods of the Periodic Table do not .The steps to draw the Lewis structure of SO4 2- are as follows: 1. Determine the total number of valence electrons in the molecule. In this case, sulfur (S) has 6 valence electrons and each oxygen (O) atom has 6 valence electrons. Since there are four oxygen atoms, the total number of valence electrons is 6 + (6 * 4) + 2 = 32.Substrate structure controls substitution mechanism S N 1 or S N 2; Stability and structure of carbocations; . Home / Gallery / [SO4]2- – Sulfate [SO 4] 2-– Sulfate. CONTROLS () How useful was this page? Click on a star to rate it! .

There are equivalent six resonance structures SO4 2- the Sulfate ion. We start with a valid Lewis structure and then follow these general rules.- Resonance .
so42- lewis structure|Iba pa
PH0 · sulfation lewis formel
PH1 · nh4+ lewis structure
PH2 · ion sulfate schéma de lewis
PH3 · ion sulfate lewis
PH4 · Iba pa